
 |
In 1856 Loschmidt qualified as a teacher and obtained a post at the Vienna Realschule. He was appointed assistant professor of physical chemistry at the University of Vienna in 1868. Loschmidt was the first to use double and triple lines to graphically represent the double and triple bonds in organic molecules. He partly explained the structures of several organic and inorganic compounds, among them benzene, toluene, and ozone, and he also recognized that an element could have several valences. Loschmidt also discovered something that was not recognised at the time. There is some controversy as to whether Loschmidt actually discovered the real structure behind Benzene or not. Kekule read a pamphlet by Loschmidt by 1862, 3 years before publishing his famed structure. In that pamphlet was a structure more or less showing a benzene molecule (see below). 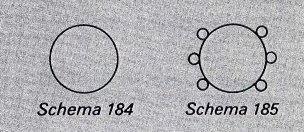 In this picture you can see there is a ring structure with 6 attached atoms around it. Pretty similar to Kekule's original "dream". This structure was out in 1861! An argument against this is that Kekule dismissed this idea in his first paper. Why attract attention to an unwanted theory?!
In this picture you can see there is a ring structure with 6 attached atoms around it. Pretty similar to Kekule's original "dream". This structure was out in 1861! An argument against this is that Kekule dismissed this idea in his first paper. Why attract attention to an unwanted theory?!
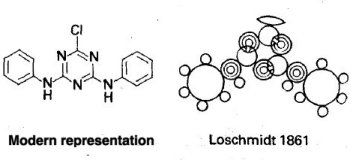 As you can see there are clearly rings, with double and triple bonds. An explanation of these bonds can be seen below. The one to look for is the middle ring. If you look closely it seems as though the two benzene rings have suddenly been written in shorthand.
As you can see there are clearly rings, with double and triple bonds. An explanation of these bonds can be seen below. The one to look for is the middle ring. If you look closely it seems as though the two benzene rings have suddenly been written in shorthand.
However if you were to transpose the symbols from the middle ring to the benzene ones, you would get the accurate structure of benzene!! 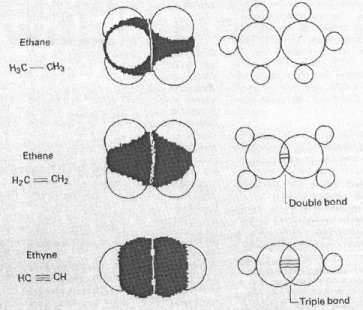 As you can see in this picture Loschmidt clearly depicts single, double and triple bonds. What is far more impressive is that each one is a different length. Now what this could relate to in the Chemistry of today is the first instance of Quantum Mechanics!
As you can see in this picture Loschmidt clearly depicts single, double and triple bonds. What is far more impressive is that each one is a different length. Now what this could relate to in the Chemistry of today is the first instance of Quantum Mechanics!
To see more of Loschmidt's diagrams taken from his booklet published in 1861 please click here. The main reason why Loschmidt is not recognised by the scientific community as discovering benzene's structure, is because he did not provide any empirical justification. Well, its intriguing anyway!! |
 |