
 |
Testing Armstrong and WynneThe Test:When the Armstrong Wynne project first started, I was curious to see chemical samples older than 100 years. As a former chemist it was interesting to be handling chemicals made over a century ago.Being in a lab nowadays entails a lot of work, but a lot of it is done by machines and specific glassware. Although work is hard in a lab it does not compare to the early 1900s. We now have glove boxes for airtight experiments, specific heating mantles for temperatures, microbalances that can work out weights down to a thousandth of a gram. In the early 1900s all this would not have existed. As you can see from the videos in the gallery section you could actually smoke in the lab! So with this in mind I used a document made by the University of Manchester that actually catalogued these chemicals to try and find any anomalies. Under the supervision of Professor Henry Rzepa I looked for any chemicals which had a melting point which would be above or below 30oc in melting point from what they should be. Scouring a 260 or so chemical list I checked for melting points against the equivalent of the Chemistry Bible known as Beilstein which has records of every published molecule from well over 200 years! Of course, this was a little test that was more of a skeptical test than anything else. It is not everyday that you can test something that is 100 years old. I mean I have a hard time in a lab doing some experiments, is it really possible to do them right without half the equipment? After about 4 days of ploughing through an interminable list, I identified 4 compounds which had melting points which were anomalous. Excellent! Mind you 4 out of 260 is still an amazing figure! The following chemicals were anomalous:
So, the question is what to do now? Well in Chemistry there is a technique known as X-Ray Crystallography. With this special process you can determine a compound’s structure through X-ray diffractions which generate a 3 dimensional image. Of course, determining a structure of a 100 year old sample requires some skill and a lot of hope that the chemical is not decomposed. I took the samples over to Dr. Andrew White, at Imperial College, who was very interested in testing out these old crystals. At first he was a little skeptical about the feasibility of the experiment because the crystals were old and a little too brittle for his liking. However he said he would give it a go and let me know. Result 1 came in:The 1,2,3 tri-chloro-naphthalene was definitely not a 1,2,3 configuration. As you can see from the diagram below, the picture shows numbered atoms. The chlorine atoms attach themselves onto the white atoms in the rings. Each atom is given a number apart from the two centre atoms which cannot form any more bonds.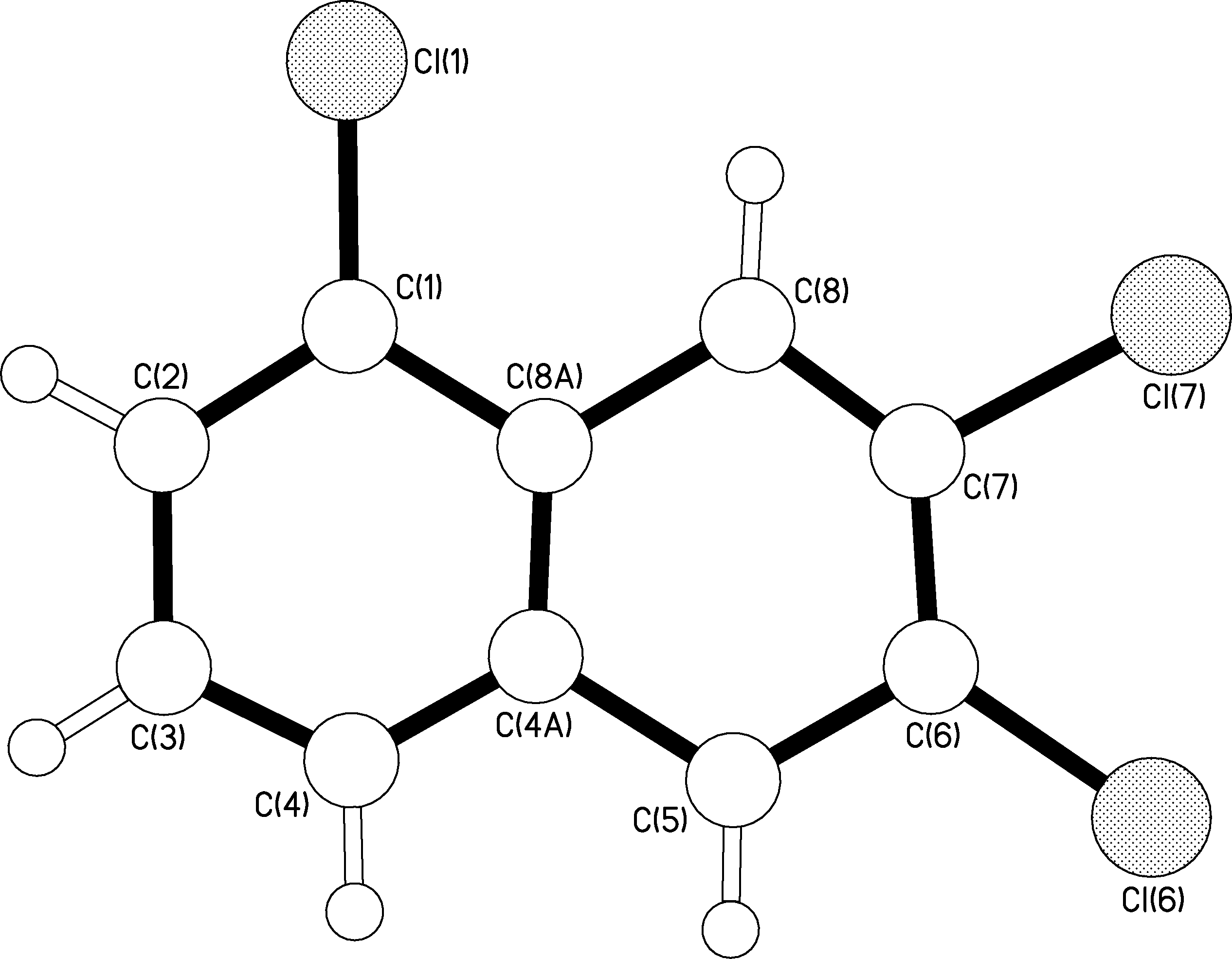
As you can see the structure you get is clearly a 1,6,7 tri-chloro-naphthalene. Excellent! I knew it was not possible. So with a small element of guilt I decided to report this first structure to Professor Henry Rzepa. Of course being a professor he was interested but cautious in our findings. The following list of questions arose:
Only one option remained. To do a re-crystallisation where you dissolve the sample into a solvent and try and extract out some pure crystals. Result 2:As you can see from the diagram it was actually spot on! On further analysis, i.e. running an extra melting point on the sample and also checking again on Beilstein, it turns out that Armstrong and Wynne reported the right melting point! Therefor the 1,2,4, tri-chloro-naphthalene picture below does represent the correct structure reported on the tube.Results 3 and 4:These two samples at first raised my suspicion. They were both the same chemical supposedly, but I could find no record of them being reported by either Armstrong or Wynne. So the question is what happened? Why on earth was there a 1,4, di-chloro-naphthalene sample tube twice in the pack with a melting point completely off what it should be?Well when the results of the crystallography came in it became fairly obvious. The samples were not what was labeled, but actually 1,5 di-chloro-naphthalene. If you then check these compounds on Beilstein you get a report from Armstrong and Wynne at the correct melting point. A further melting point then confirmed that these were in fact 1,5 di-chloro-naphthalenes. So what have we got so far?Well so far we have one out of three anomalies unaccounted for. The first chemical sample, the 1,2,3 substituted naphthalene is wrong. The melting point is wrong and for some reason Wynne reports such a structure in a journal.As I tried to re-crystallise some of the sample to get a better batch of crystals something hit me. Why was there a report from Wynne but not Armstrong in Beilstein about 1,2,3 tri-chloro-naphthalene? Looking further into the matter revealed something interesting. It turns out that the report in a journal was actually reported in 1941. Armstrong passed away in 1937, so something could not be right. Either this chemical sample was not meant to be in the collection or something else was wrong. Was the sample mislabeled? That would explain a lot. Now this is where a sudden realization hit me. If you take a careful look at the picture below, you can see that the two rings are opposite and symmetrical to each other. That means that you can number the atoms left to right or right to left. 
That would mean that you would have two sets of rings with numbers from 1 through to 4 as shown below. 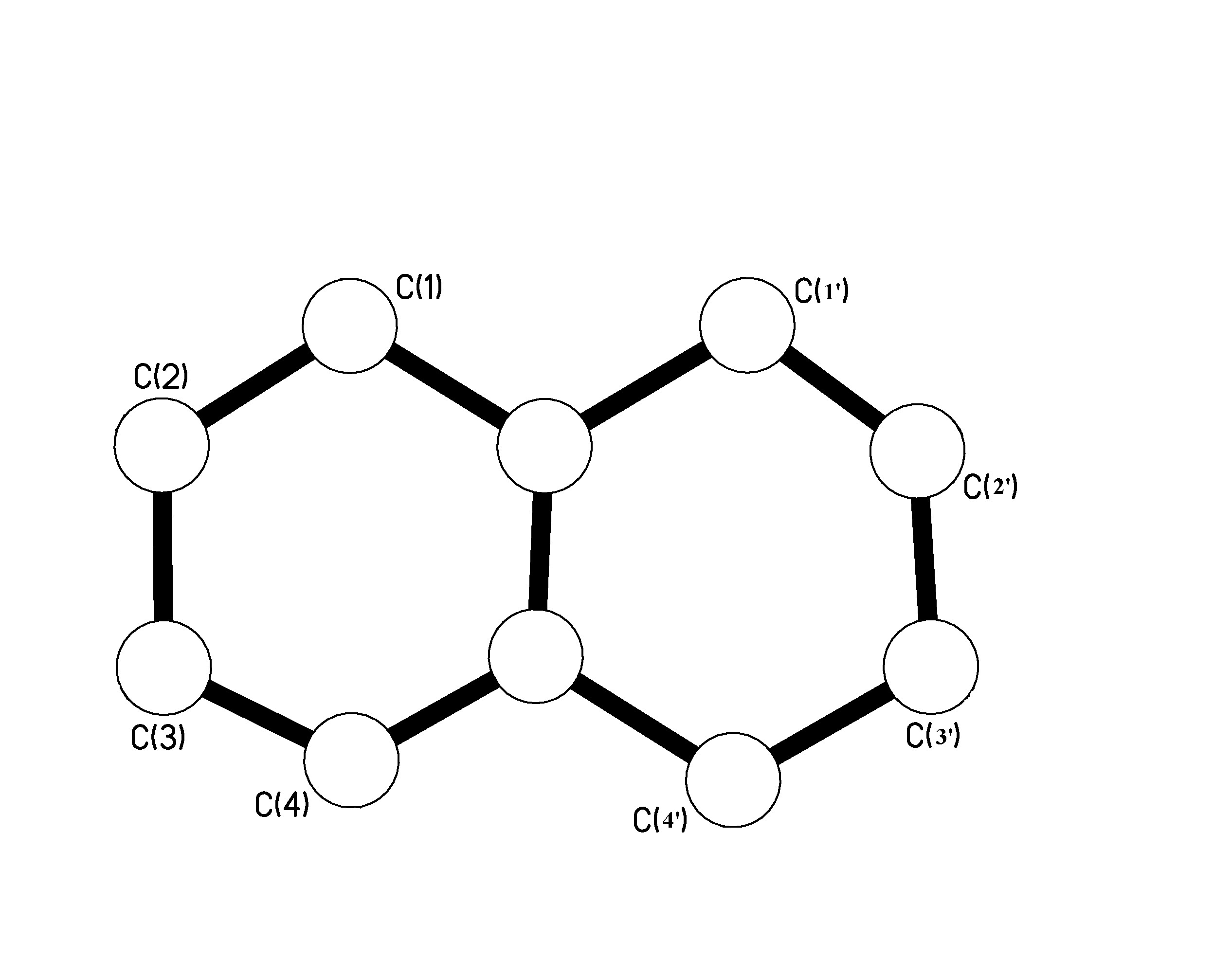
So if we re-draw the structure with the new number configuration you get something known as 1,2',3’. The ‘ is the key in this story! This was a notation meaning prime. In other words the 2' and 3‘ were actually molecules on the second ring. If you look at the diagram below you can see it perfectly matches the 1,6,7 configuration of the crystallographic results. Surprisingly enough, the melting point of the sample concurs with the 1,6,7 melting point! Taking a closer look at the sample label, it turns out there is a 3’ which is not that easy to read. I guess I should get some glasses! Conclusion:Armstrong and Wynne synthesized these chemicals over 100 years ago. This was not a test to show that they could be wrong. This was a small analysis to prove the worth of their chemistry. Armstrong and Wynne did a huge amount of work based on substitution reactions, which are incredibly important to the dyestuffs industry.The 4 samples we took were anomalous on paper, but it was a human error made many years later that sparked controversy. I am personally relieved that nothing turned up; two scientists who have devoted their lives to a project deserve the respect. Armstrong and Wynne synthesized these chemicals in labs kitted out with nothing more than some test tubes, Bunsen burners and basic equipment. The purity of their samples is incredible, in each case the samples withstood the test of time. I hope that if any chemist is reading this report that you never ever complain about your equipment again! |
 |